It's really hard how to find out how much density an object has, right? Wrong. It's really quite easy. All you have to do is use the formula of density: density=mass/volume. A very easy way to remember this is to think of a broken heart. The "M" for mass, divided by a "V" for volume creates the shape of a heart cut in half by a division symbol. If an object or certain material is more dense than the molecules of that object or material are tightly packed together, and if a object has a lower density than the molecules are less closely packed together.
 |
| (Lead has a very high density) |
 |
| (Wood has a very low density) |
 |
| (Density formula picture) |
To find the density of an object you need to first find the volume of that object. There are a few different ways to do this, If the object is a regular shape than you can mesure it, if the object is irregular than you use the water displacement method. When you are finding the volume of an object by measuring it, for example a cube. You need to find three different measurements, the length of the cube, the width of the cube and hight of the cube, this can be remembered as LxWxH.
 |
| (http://upload.wikimedia.org/wikipedia/commons/e/ec/IsometricCubeGray.png) |
When calculating the volume of an irregular object using water displacement you need a few different materials. A graduated cylinder, water and of course the object. When you are finding the volume using water displacement there is one key thing that you have to keep in mind, the meniscus of the water in the graduated cylinder. A meniscus is a crescent shape on the upper surface of a liquid. When measuring how far the water has risen you have to always mesure to the bottom of the meniscus.
 |
| (http://www.esu.edu/~scady/07424717.jpg) |
A rock is an example of a irregularly shaped object. When trying to find the volume of a rock you have to use the water displacement method. You first put the rock into a graduated cylinder full of water. To find the volume of the rock you see how far the water rose when the rock was put into the water. For example, if the water level started at 10ml and when the rock was placed into the water the water level went up to 15ml, than the volume of the rock is 5ml.
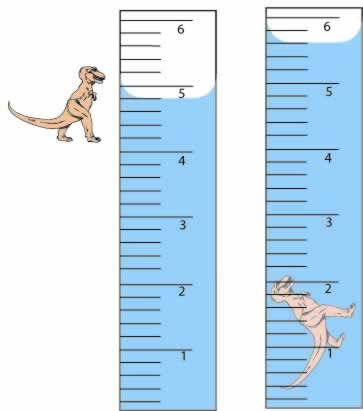 |
| (http://www.cstephenmurray.com/onlinequizes/chemistry/measuring/Dinocopy.jpg) |
While trying to understand density we did the "Mystery Liquids-Rainbow straws lab". During the lab we tried to find the order of how dense all the liquids were. To do this we had to put two different liquids into a small straw and find which one was denser. We then took the liquid that was more dense and compared it to another liquid to see the more dense liquid out of those two. We continued to do this until we found the order of the liquids from least dense to most dense.
 |
| (http://density-whatitmeans.wikispaces.com/file/view/density_layers/285963846/240x299/density_layers) |
Another lab that we did was the "Density Bottles Lab". In this lab we were given a bottle with different colored beads inside and what at first looked like water surrounding all the beads. When the bottle is first shook all of the beads mix together in a random order, but after a few seconds they start to settle down and the blue beads went to the bottom of the bottle and the white beads went to the top. As you continue to wait both colors of beads start to move to the center. The beads eventually come together in the very middle of the bottle, the white beads on the top and the blue beads on the bottom. When we look at this more closely we see that there is not just water in the bottle but also another liquid (Rubbing alcohol) that has a density that is almost the exact same as the density of water but is just a little bit less dense. This lab helps us learn a couple different things. One, that rubbing alcohol is less dense than water, and two, that the material on top is the least dense, while the material on the bottom is the most dense.
 |
| (http://cdn.teachersource.com/images/products/pop/den460.jpg) |
A third lab we did during the density unit is the "lava Lamp Density Exploration Lab". This lab was ment to show us that when a something is heated it becomes less dense and when something cools down it becomes more dense. Inside of a lava lamp there is a small light, this light heats the lava and makes the lava rise to the top of the lamp. When the lava at the top of the lamp starts to cool down it starts to go back down towards the heat source again. when the colder lava comes into contact with the heat again it starts to rise again creating a convection current of the lava moving in a circle.
 |
| (http://www.spencersonline.com/images/spencers/products/interactivezoom/processed/00530790.interactive.a.jpg) |









No comments:
Post a Comment
Note: Only a member of this blog may post a comment.